How to Optimize Tablet Manufacturing for Increased Efficiency and Quality
In the rapidly evolving field of tablet manufacturing, the demand for increased efficiency and superior quality has never been more paramount. According to a recent industry report by Smith & Associates, the tablet market is projected to grow at a compound annual growth rate (CAGR) of 6.2% through 2025, highlighting the critical need for manufacturers to optimize their production processes. Industry analysts emphasize that leveraging advanced technologies and data analytics can significantly streamline operations, reduce waste, and enhance overall product quality.
Expert insight from Dr. Emily Tran, a leading specialist in tablet manufacturing processes, reinforces this necessity: "To thrive in the competitive landscape of tablet production, companies must invest in innovative manufacturing techniques that prioritize efficiency and quality. Implementing automation and real-time monitoring can lead to substantial gains in productivity." As manufacturers strive to meet the increasing consumer expectations and regulatory standards, a strategic focus on optimizing production lines becomes essential for long-term success. By embracing continuous improvement practices, the tablet manufacturing industry can not only enhance profitability but also ensure a sustainable future in a global marketplace.

Understanding the Tablet Manufacturing Process and Its Challenges
The tablet manufacturing process is intricate, involving various stages that require precision and coordination to ensure both efficiency and quality. Key phases include formulation, granulation, compression, and coating. According to a recent industry report by MarketsandMarkets, the global tablet manufacturing market is projected to grow from $25 billion in 2022 to $36 billion by 2027, driven by rising demand for cost-effective medication delivery methods. Challenges such as scaling production without compromising quality and managing supply chain disruptions have become increasingly prominent. Identifying bottlenecks in the manufacturing chain is imperative for optimizing workflow and reducing lead times.
Another significant challenge lies in maintaining consistent product quality throughout the entire manufacturing process. A study published in the Journal of Pharmaceutical Sciences indicated that issues such as material variability and equipment downtime can lead to a decrease in overall production quality. The adoption of advanced technologies like real-time monitoring and predictive maintenance can help mitigate these risks. Industry professionals emphasize the importance of adhering to Good Manufacturing Practices (GMP) to uphold product integrity and compliance, thereby fostering consumer trust and ensuring regulatory adherence. As manufacturers navigate these challenges, leveraging data analytics and automation will be crucial in enhancing productivity while ensuring high standards of quality.
Key Factors Influencing Efficiency in Tablet Production
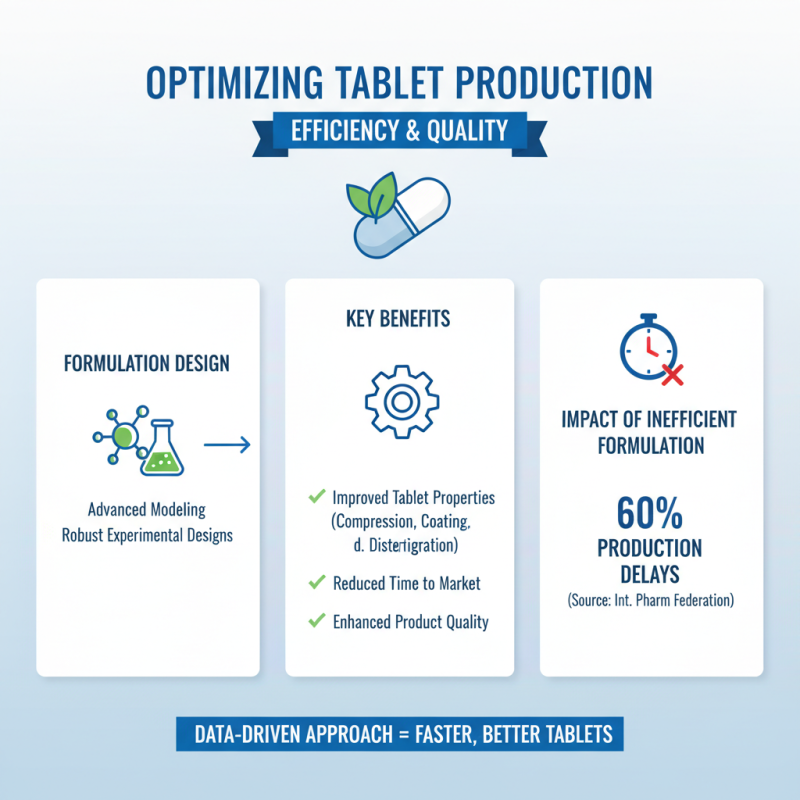
In the realm of pharmaceutical manufacturing, optimizing tablet production for increased efficiency and quality hinges on several key factors. One significant influence is the formulation design and processing parameters. According to a report from the International Pharmaceutical Federation, approximately 60% of production delays can be attributed to inefficiencies in formulation development. By employing advanced modeling techniques and robust experimental designs, manufacturers can streamline their formulation processes, leading to better compression, coating, and disintegration properties in tablets. This not only enhances product quality but also reduces the time to market for new formulations.
Another crucial factor affecting efficiency in tablet production is the technology utilized in manufacturing processes. The implementation of continuous manufacturing systems has been shown to increase production efficiency by 30% compared to traditional batch processing methods. A study published in the Journal of Pharmaceutical Innovation emphasized that continuous processes allow for real-time monitoring and control, which minimizes variability and enhances product consistency. Moreover, integrating automation and data analytics can lead to predictive maintenance of equipment, thus reducing downtime and further optimizing production workflows. By focusing on these critical factors, manufacturers can significantly elevate both efficiency and quality in tablet production.
Implementing Quality Control Measures in Tablet Manufacturing
In tablet manufacturing, implementing robust quality control measures is crucial for enhancing both efficiency and product quality. According to a report by the International Society for Pharmaceutical Engineering (ISPE), nearly 40% of production defects can be traced back to inadequate quality control processes. By integrating quality control at every step of the manufacturing process—from raw material inspection to final product testing—manufacturers can significantly reduce waste and increase overall yield. This proactive approach not only meets regulatory standards but also boosts consumer confidence in the product's reliability and safety.
Automation and data analytics play a pivotal role in optimizing quality control in tablet manufacturing. A study by Frost & Sullivan indicates that manufacturers employing advanced analytics can improve defect detection rates by up to 30%. This can be achieved through real-time monitoring of critical parameters such as tablet weight and hardness, which directly influence the product's efficacy. By adopting these technologies, companies can swiftly identify and rectify deviations, leading to a consistent output that aligns with quality expectations. Strengthening quality control measures through automation and data-driven insights ultimately paves the way for improved operational efficiency, reducing both operational costs and time to market.
Technological Innovations for Enhanced Tablet Production

Technological innovations greatly enhance tablet production, making processes more efficient and ensuring higher quality products. Advanced automation technologies, such as robotics and artificial intelligence, streamline operations by minimizing human error and increasing production speeds. Robotics can handle repetitive tasks with precision, while AI algorithms can analyze production data in real-time to optimize workflows and identify potential bottlenecks. This combination not only boosts throughput but also allows manufacturers to adapt swiftly to changing market demands.
Furthermore, the integration of sophisticated software systems plays a crucial role in enhancing quality control during tablet manufacturing. These systems use machine learning to monitor production processes, ensuring consistency in the formulation and physical properties of the tablets. By employing sensors and data analytics, manufacturers can assess the quality at every stage, from formulation to final packaging. This proactive approach helps in identifying defects early, ultimately reducing waste and improving overall product quality. The synergy of these technological advancements creates a responsive and efficient manufacturing environment that is well-suited for the fast-paced pharmaceutical industry.
Training and Development for Skilled Workforce in Tablet Manufacturing
In the realm of tablet manufacturing, the role of a skilled workforce cannot be overstated. Training and development initiatives are essential in equipping employees with the necessary skills and knowledge to enhance operational efficiency and ensure product quality. A comprehensive training program should focus on both technical competencies and soft skills. Technical training encompasses understanding manufacturing processes, equipment operation, and quality control practices, while soft skills such as teamwork, communication, and problem-solving are crucial for fostering a cohesive work environment.
To create a more adept workforce, manufacturers should implement continuous learning opportunities, such as workshops, online courses, and hands-on training sessions. Emphasizing the importance of staying updated with the latest advancements in technology and industry standards is vital. Moreover, mentorship programs can bridge the knowledge gap between experienced workers and new hires, facilitating knowledge transfer and promoting a culture of continuous improvement. When employees feel valued and confident in their abilities, they are more likely to contribute positively to the manufacturing process, ultimately leading to increased efficiency and enhanced product quality.
How to Optimize Tablet Manufacturing for Increased Efficiency and Quality
| Dimension | Description | Current Value | Target Value |
|---|---|---|---|
| Production Cycle Time | Time taken to produce a batch of tablets | 5 hours | 4 hours |
| Yield Rate | Percentage of tablets produced meeting quality standards | 85% | 95% |
| Employee Training Hours | Hours invested in workforce training per employee per month | 10 hours | 15 hours |
| Equipment Downtime | Time when machinery is not operational | 12% | 5% |
| Customer Satisfaction Score | Measured satisfaction levels from customer feedback | 78% | 90% |
Related Posts
-

How to Choose the Best Film Coating for Your Projects and Applications
-
How to Choose the Best Tablet Coating for Optimal Protection and Longevity
-

What is Pharmaceutical Coating and How Does It Impact Drug Delivery
-
Top 10 Tablet Coating Systems for Improved Pharmaceutical Production Efficiency
-

Top 10 Benefits of Using a Tablet Coating Pan for Efficient Coating Process
